Abstract
Review Article
SARS-CoV-2 Infection, COVID-19, and long covid: Saga of erratic immune response, waning immunity, and immune system failure
Vinod Nikhra*
Published: 09 August, 2021 | Volume 5 - Issue 1 | Pages: 078-087
Introduction - evolution of SARS-CoV-2 variants: With the unrestrained pandemic for over last one-and-half year, SARS-CoV-2 seems to have adapted to its habitat, the human host, through mutations that facilitate its replication and transmission. The G variant incorporating D614G mutation, potently more transmissible than the ancestral virus arose during January 2020 and spread widely. Since then, various SARS-CoV-2 variants of concern (VOCs) and variants of interest (VOIs) with higher infectivity or virulence or both, have evolved on the background of G variant, and spread widely.
SARS-CoV-2 infection and the immunodynamics: As the virus becomes more transmissible, its lethality may drop. Apart from the humoral immunity, T-cell recognition from a previous SARS-CoV-2 infection or vaccination may modify the disease transmission correlates and its clinical manifestations. On the other hand, the immunity generated may reduce probability of re-infection as well as limit evolution of adaptive mutations, and emergence of highly infectious and immune-escape variants. There are complex issues related to the SARS-CoV-2 evolutionary dynamics and host’s immunodynamics.
Trending etiopathoimmunological correlates: The evolution potential of SARS-CoV-2 is limited because of proofreading function of nsp14. The S protein mutations affect transmissibility, virulence, and vaccine efficacy. The D614G mutation in G variant with higher infectivity has turned the Chinese epidemic into a pandemic. Other SARS-CoV-2 variants, such as Alpha, Beta, Gamma, and Delta seem to have evolved as result of adaptation to selective pressures during periods of prolonged infections and subsequent transmission. Further, there is issue of convergent association of mutations.
Basics of immunity and immune system failure: The nature of the immune response after natural SARS-CoV-2 infection is variable and diverse. There are pre-existing neutralizing antibodies and sensitized T cells elicited during previous infection with seasonal CoVs influencing the disease susceptibility and course. The virus has evolved adaptive mechanisms to reduce its exposure to IFN-I and there are issues related to erratic and overactive immune response. The altered neutralizing epitopes in the S protein in SARS-CoV-2 variants modify the immune landscapes and clinical manifestations.
Conclusion: current scenarios and prospects: Presently, the SARS-CoV-2 infection is widespread with multiple evolving infectious variants. There is probability of its transition from epidemic to endemic phase in due course manifesting as a mild disease especially in the younger population. Conversely, the pandemic may continue with enhanced disease severity due to evolving variants, expanded infection pool, and changing immunity landscape. There is need to plan for the transition and continued circulation of the virus during the endemic phase or continuing pandemic for indefinite period.
Read Full Article HTML DOI: 10.29328/journal.jprr.1001030 Cite this Article Read Full Article PDF
Keywords:
COVID immune landscape; Convergent mutations; D614G G variant; Erratic and overactive response; Immune escape; Intra-host diversity; Variants of concern (VOCs); Variants of interest (VOIs); Vaccine upgradation
References
- Hou YJ, Chiba S, Halfmann P, Ehre C, Kuroda M, et al. SARS-CoV-2 D614G variant exhibits efficient replication ex vivo and transmission in vivo. Science. 2020; 370: 1464-1468. PubMed: https://pubmed.ncbi.nlm.nih.gov/33184236/
- Volz E, Hill V, McCrone JT, Price A, Jorgensen D, et al. Evaluating the Effects of SARS-CoV-2 Spike Mutation D614G on Transmissibility and Pathogenicity. Cell. 2021; 184: 64-75. PubMed: https://pubmed.ncbi.nlm.nih.gov/33275900/
- Korber B, Fischer WM, Gnanakaran S, Yoon H, Theiler J, et al. Sheffield COVID-19 Genomics Group. Tracking changes in SARS-CoV-2 spike: evidence that D614G increases infectivity of the COVID-19 virus. Cell. 2020; 182: 812-827.e19. PubMed: https://pubmed.ncbi.nlm.nih.gov/32697968/
- Guzzi PH, Mercatelli D, Ceraolo C, Giorgi FM. Master Regulator Analysis of the SARS-CoV-2/Human Interactome. J Clin Med. 2020; 9: 982. PubMed: https://pubmed.ncbi.nlm.nih.gov/32244779/
- Starr TN, Greaney AJ, Dingens AS, Bloom JD. Complete map of SARS-CoV-2 RBD mutations that escape the monoclonal antibody LY-CoV555 and its cocktail with LY-CoV016. Cell Reports Medicine. 2021; 2: 100255.
- The Public Health England. https://www.gov.uk/government/publications/investigation-of-sars-cov-2-variants-of-concern-routine-variant-data-update
- Levine-Tiefenbrun M, Yelin I, Katz R, Herzel E, Golan Z, et al. Decreased SARS-CoV-2 viral load following vaccination. Medrxiv Preprint. 2021.
- Cobey S, Larremore DB, Grad YH, Lipsitch M. Concerns about SARS-CoV-2 evolution should not hold back efforts to expand vaccination. Nat Rev Immunol. 2021; 21: 330-335. PubMed: https://pubmed.ncbi.nlm.nih.gov/33795856/
- Saad-Roy CM, Morris SE, Metcalf CJE, Mina MJ, Baker RE, et al. Epidemiological and evolutionary considerations of SARS-CoV-2 vaccine dosing regimes. Science. 2021; 372: 363-370. PubMed: https://pubmed.ncbi.nlm.nih.gov/33688062/
- He X, Lau EHY, Wu P, Deng X, Wang J, et al. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat Med. 2020; 26: 672-675. PubMed: https://pubmed.ncbi.nlm.nih.gov/32296168/
- Hanage WP, Russell CA. Partial immunity and SARS-CoV-2 mutations. Science. 2021; 372: 354. PubMed: https://pubmed.ncbi.nlm.nih.gov/33888632/
- Martin MA, VanInsberghe D, Koelle K. Insights from SARS-CoV-2 sequences. Science. 2021: 371: 466-467. PubMed: https://pubmed.ncbi.nlm.nih.gov/33510015/
- McCormick KD, Jacobs JL, Mellors JW. The emerging plasticity of SARS-CoV-2. Science. 2021; 371: 1306-1308. PubMed: https://pubmed.ncbi.nlm.nih.gov/33766871/
- Starr TN, Greaney AJ, Hilton SK, Ellis D, Crawford KHD, et al. Deep Mutational Scanning of SARS-CoV-2 Receptor Binding Domain Reveals Constraints on Folding and ACE2 Binding. Cell. 2020; 182: 1295-1310. PubMed: https://pubmed.ncbi.nlm.nih.gov/32841599/
- Avanzato VA, Matson MJ, Seifert SN, Pryce R, Williamson BN, et al. Case Study: Prolonged Infectious SARS-CoV-2 Shedding from an Asymptomatic Immunocompromised Individual with Cancer. Cell. 2020; 183: 1901-1912. PubMed: https://pubmed.ncbi.nlm.nih.gov/33248470/
- Baang JH, Smith C, Mirabelli C, Valesano AL, Manthei DM, et al. Prolonged Severe Acute Respiratory Syndrome Coronavirus 2 Replication in an Immunocompromised Patient. J Infect Dis. 2021; 223: 23-27. PubMed: https://pubmed.ncbi.nlm.nih.gov/33089317/
- Camprubí D, Gaya A, Marcos MA, Martí-Soler H, Soriano A, et al. Persistent replication of SARS-CoV-2 in a severely immunocompromised patient treated with several courses of remdesivir. Int J Infect Dis. 2021; 104: 379-381. PubMed: https://pubmed.ncbi.nlm.nih.gov/33359065/
- Hensley MK, Bain WG, Jacobs J, Nambulli S, Parikh U, et al. Intractable COVID-19 and Prolonged SARS-CoV-2 Replication in a CAR-T-cell Therapy Recipient: A Case Study. Clin Infect Dis. 2021; 73: e815-e821. PubMed: https://pubmed.ncbi.nlm.nih.gov/33507235/
- Kemp SA, Collier DA, Datir RP, Ferreira IATM, Gayed S, et al. SARS-CoV-2 evolution during treatment of chronic infection. Nature. 2021; 592: 277-282. PubMed: https://pubmed.ncbi.nlm.nih.gov/33545711/
- Choi B, Choudhary MC, Regan J, Sparks JA, Padera RF, et al. Persistence and Evolution of SARS-CoV-2 in an Immunocompromised Host. N Engl J Med. 2020; 383: 2291-2293. PubMed: https://pubmed.ncbi.nlm.nih.gov/33176080/
- Liu C, Ginn HM, Dejnirattisai W, Supasa P, Wang B, et al. Reduced neutralization of SARS-CoV-2 B.1.617 by vaccine and convalescent serum. Cell. 2021; 184: 1–17.
- Wang P, Casner RG, Nair MS, Wang M, Yu J, et al. Increased resistance of SARS-CoV-2 variant P.1 to antibody neutralization. Cell Host Microbe. 2021; 29: 747–751. PubMed: https://pubmed.ncbi.nlm.nih.gov/33887205/
- Planas D, Bruel T, Grzelak L, Guivel-Benhassine F, Staropoli I, et al. Sensitivity of infectious SARS-CoV-2 B.1.1.7 and B.1.351 variants to neutralizing antibodies. Nat Med. 2021; 27: 917–924. PubMed: https://pubmed.ncbi.nlm.nih.gov/33772244/
- WHO – Variants of Concern and Variants of Interest. 2021. https://www.who.int/en/activities/tracking-SARS-CoV-2-variants
- Bugembe DL, Phan MVT, Ssewanyana I. A SARS-CoV-2 lineage A variant (A.23.1) with altered spike has emerged and is dominating the current Uganda epidemic. medRixv Preprint. 2021.
- Updated 2021, CDC, USA. https://www.cdc.gov/coronavirus/2019-ncov/variants/variant-info.html.
- Self WH, Tenforde MW, Stubblefield WB, Feldstein LR, Steingrub JS, et al. Decline in SARS-CoV-2 Antibodies After Mild Infection Among Frontline Health Care Personnel in a Multistate Hospital Network — 12 States. April–August 2020. MMWR Morb Mortal Wkly Rep. 2020; 69: 1762–1766. PubMed: https://pubmed.ncbi.nlm.nih.gov/33237893/
- Yamaguchi T, Shinagawa T, Kobata H, Nakagawa H. Immunity against seasonal human coronavirus OC43 mitigates fatal deterioration of COVID-19. Int J Infect Dis. 2021; 109: 261-268. PubMed: https://pubmed.ncbi.nlm.nih.gov/34273512/
- Peiris M, Leung GM. What can we expect from first-generation COVID-19 vaccines? The Lancet. 2020; 396: 1467-1469. PubMed: https://pubmed.ncbi.nlm.nih.gov/32971042/
- Le Bert N, Tan AT, Kunasegaran K, Tham CYL, Hafezi M, et al. SARS-CoV-2-specific T cell immunity in cases of COVID-19 and SARS, and uninfected controls. Nature. 2020; 584: 457–462.
- Beretta A, Cranage M, Zipeto D. Is Cross-Reactive Immunity Triggering COVID-19 Immunopathogenesis? Front Immunol. 2020; 11: 567710. PubMed: https://pubmed.ncbi.nlm.nih.gov/33178193/
- Weisblum Y, Schmidt F, Zhang F, DaSilva J, Poston D, et al. Escape from neutralizing antibodies by SARS-CoV-2 spike protein variants. Elife. 2020; 9: e61312. PubMed: https://pubmed.ncbi.nlm.nih.gov/33112236/
- Sa Ribero M, Jouvenet N, Dreux M, Nisole S. Interplay between SARS-CoV-2 and the type I interferon response. PLoS Pathog. 2020; 16: e1008737. PubMed: https://pubmed.ncbi.nlm.nih.gov/32726355/
- Felsenstein S, Herbert JA, McNamara PS, Hedrich CM. COVID-19: Immunology and treatment options. Clin Immunol. 2020; 215: 108448. PubMed: https://pubmed.ncbi.nlm.nih.gov/32353634/
- Bolouri H, Speake C, Skibinski D, Long SA, Hocking AM, et al. The COVID-19 immune landscape is dynamically and reversibly correlated with disease severity. J Clin Invest. 2021; 131: e143648. PubMed: https://pubmed.ncbi.nlm.nih.gov/33529167/
- Zhao Y, Kilian C, Turner JE, Bosurgi L, Roedl K, et al. Clonal expansion and activation of tissue-resident memory-like Th17 cells expressing GM-CSF in the lungs of severe COVID-19 patients. Sci Immunol. 2021; 6: eabf6692. PubMed: https://pubmed.ncbi.nlm.nih.gov/33622974/
- Karki R, Sharma BR, Tuladhar S, Williams EP, Zalduondo L, et al. COVID-19 cytokines and the hyperactive immune response: Synergism of TNF-α and IFN-γ in triggering inflammation, tissue damage, and death. Cell. 2021; 184: 149-168.
- Hossain MK, Hassanzadeganroudsari M, Apostolopoulos V. The emergence of new strains of SARS-CoV-2. What does it mean for COVID-19 vaccines? Expert Rev Vaccines. 2021; 1-4. PubMed: https://pubmed.ncbi.nlm.nih.gov/33896316/
- Lavine JS, Bjornstad ON, Antia R. Immunological characteristics govern the transition of COVID-19 to endemicity. Science. 2021; 371: 741-745. PubMed: https://pubmed.ncbi.nlm.nih.gov/33436525/
- Altmann DM, Boyton RJ, Beale R. Immunity to SARS-CoV-2 variants of concern. Science. 2021; 371: 1103-1104. PubMed: https://pubmed.ncbi.nlm.nih.gov/33707254/
- Yuen KS, Ye ZW, Fung SY, Chan CP, Jin DY. SARS-CoV-2 and COVID-19: The most important research questions. Cell Biosci. 2020; 10: 40.
- Saad-Roy CM, Wagner CE, Baker RE, Morris SE, Farrar J, et al. Immune life history, vaccination, and the dynamics of SARS-CoV-2 over the next 5 years. Science. 2020; 370: 811-818. PubMed: https://pubmed.ncbi.nlm.nih.gov/32958581/
- Lythgoe KA, Hall M, Ferretti L, de Cesare M, MacIntyre-Cockett G, et al. SARS-CoV-2 within-host diversity and transmission. Science. 2021; 372: eabg0821. PubMed: https://pubmed.ncbi.nlm.nih.gov/33688063/
- Peng Y, Mentzer AJ, Liu G, Yao X, Yin Z, et al. Broad and strong memory CD4+ and CD8+ T cells induced by SARS-CoV-2 in UK convalescent individuals following COVID-19. Nat Immunol. 2020; 21: 1336-1345. PubMed: https://pubmed.ncbi.nlm.nih.gov/32887977/
- Abu-Raddad LJ, Chemaitelly H, Adeel A. Butt AA. Effectiveness of the BNT162b2 Covid-19 Vaccine against the B.1.1.7 and B.1.351 Variants. N Engl J Med. 2021; 385: 187-189. PubMed: https://pubmed.ncbi.nlm.nih.gov/33951357/
- Gómez CE, Perdiguero B, Esteban M. Emerging SARS-CoV-2 Variants and Impact in Global Vaccination Programs against SARS-CoV-2/COVID-19. Vaccines (Basel). 2021; 9: 243. PubMed: https://pubmed.ncbi.nlm.nih.gov/33799505/
- Benefits of Getting a COVID-19 Vaccine. Center for Disease control and Prevention. 2021. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/vaccine-benefits.html
- Mysterious Ailment, Mysterious Relief: Vaccines Help Some COVID Long-Haulers. 2021. https://www.npr.org/sections/health-shots/2021/03/31/982799452/mysterious-ailment-mysterious-relief-vaccines-help-some-covid-long-haulers
- Sironi M, Hasnain SE, Rosenthal B, Phan T, Luciani F, et al. SARS-CoV-2 and COVID-19: A genetic, epidemiological, and evolutionary perspective. Infect Genet Evol. 2020; 84: 104384. PubMed: https://pubmed.ncbi.nlm.nih.gov/32473976/
- Plante JA, Mitchell BM, Plante KS, Debbink K, Weaver SC, et al. The variant gambit: COVID-19's next move. Cell Host Microbe. 2021; 29: 508-515. PubMed: https://pubmed.ncbi.nlm.nih.gov/33789086/
- Cooper V. The Coronavirus Variants Don’t Seem to Be Highly Variable So Far: SARS-CoV-2 may be settling into a limited set of mutations. Scientific American. 2021. https://www.scientificamerican.com/article/the-coronavirus-variants-dont-seem-to-be-highly-variable-so-far
Figures:
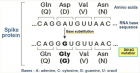
Figure 1

Figure 2
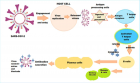
Figure 3

Figure 4
Similar Articles
-
SARS-CoV-2 Infection, COVID-19, and long covid: Saga of erratic immune response, waning immunity, and immune system failureVinod Nikhra*. SARS-CoV-2 Infection, COVID-19, and long covid: Saga of erratic immune response, waning immunity, and immune system failure. . 2021 doi: 10.29328/journal.jprr.1001030; 5: 078-087
Recently Viewed
-
FITT-CORRECT: Updated dynamic and evidence-based principle of exercise prescriptionShambhu P Adhikari*,Jarugool Tretriluxana,Rubee Dev,Emily Eglitis,Nistha Shrestha,Cheryl Kerfeld6. FITT-CORRECT: Updated dynamic and evidence-based principle of exercise prescription. J Nov Physiother Rehabil. 2021: doi: 10.29328/journal.jnpr.1001039; 5: 005-009
-
Cystoid Macular Oedema Secondary to Bimatoprost in a Patient with Primary Open Angle GlaucomaKonstantinos Kyratzoglou*,Katie Morton. Cystoid Macular Oedema Secondary to Bimatoprost in a Patient with Primary Open Angle Glaucoma. Int J Clin Exp Ophthalmol. 2025: doi: 10.29328/journal.ijceo.1001059; 9: 001-003
-
Sex after Neurosurgery–Limitations, Recommendations, and the Impact on Patient’s Well-beingMor Levi Rivka*, Csaba L Dégi. Sex after Neurosurgery–Limitations, Recommendations, and the Impact on Patient’s Well-being. J Neurosci Neurol Disord. 2024: doi: 10.29328/journal.jnnd.1001099; 8: 064-068
-
Physiotherapy Undergraduate Students’ Perception About Clinical Education; A Qualitative StudyPravakar Timalsina*,Bimika Khadgi. Physiotherapy Undergraduate Students’ Perception About Clinical Education; A Qualitative Study. J Nov Physiother Rehabil. 2024: doi: 10.29328/journal.jnpr.1001063; 8: 043-052
-
Clinical Significance of Anterograde Angiography for Preoperative Evaluation in Patients with Varicose VeinsYi Liu,Dong Liu#,Junchen Li#,Tianqing Yao,Yincheng Ran,Ke Tian,Haonan Zhou,Lei Zhou,Zhumin Cao*,Kai Deng*. Clinical Significance of Anterograde Angiography for Preoperative Evaluation in Patients with Varicose Veins. J Radiol Oncol. 2025: doi: 10.29328/journal.jro.1001073; 9: 001-006
Most Viewed
-
Evaluation of Biostimulants Based on Recovered Protein Hydrolysates from Animal By-products as Plant Growth EnhancersH Pérez-Aguilar*, M Lacruz-Asaro, F Arán-Ais. Evaluation of Biostimulants Based on Recovered Protein Hydrolysates from Animal By-products as Plant Growth Enhancers. J Plant Sci Phytopathol. 2023 doi: 10.29328/journal.jpsp.1001104; 7: 042-047
-
Sinonasal Myxoma Extending into the Orbit in a 4-Year Old: A Case PresentationJulian A Purrinos*, Ramzi Younis. Sinonasal Myxoma Extending into the Orbit in a 4-Year Old: A Case Presentation. Arch Case Rep. 2024 doi: 10.29328/journal.acr.1001099; 8: 075-077
-
Feasibility study of magnetic sensing for detecting single-neuron action potentialsDenis Tonini,Kai Wu,Renata Saha,Jian-Ping Wang*. Feasibility study of magnetic sensing for detecting single-neuron action potentials. Ann Biomed Sci Eng. 2022 doi: 10.29328/journal.abse.1001018; 6: 019-029
-
Pediatric Dysgerminoma: Unveiling a Rare Ovarian TumorFaten Limaiem*, Khalil Saffar, Ahmed Halouani. Pediatric Dysgerminoma: Unveiling a Rare Ovarian Tumor. Arch Case Rep. 2024 doi: 10.29328/journal.acr.1001087; 8: 010-013
-
Physical activity can change the physiological and psychological circumstances during COVID-19 pandemic: A narrative reviewKhashayar Maroufi*. Physical activity can change the physiological and psychological circumstances during COVID-19 pandemic: A narrative review. J Sports Med Ther. 2021 doi: 10.29328/journal.jsmt.1001051; 6: 001-007

HSPI: We're glad you're here. Please click "create a new Query" if you are a new visitor to our website and need further information from us.
If you are already a member of our network and need to keep track of any developments regarding a question you have already submitted, click "take me to my Query."