More Information
Submitted: August 12, 2023 | Approved: September 22, 2023 | Published: September 25, 2023
How to cite this article: Ntertsos N, Christantoniou G, Kyrka K, Pezirkianidou P, Bikos V, et al. Clinical Approach to Immunotherapy-induced Type 1 Diabetes Mellitus: A Case of Pembrolizumab Associated Insulin-dependent Diabetes in a Patient with NSCLC. J Pulmonol Respir Res. 2023; 7: 024-027.
DOI: 10.29328/journal.jprr.1001047
Copyright License: © 2023 Ntertsos N, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Immunotherapy; Pembrolizumab; Type 1 diabetes mellitus; Non-small cell lung cancer; Insulin; Diabetic ketoacidosis
Clinical Approach to Immunotherapy-induced Type 1 Diabetes Mellitus: A Case of Pembrolizumab Associated Insulin-dependent Diabetes in a Patient with NSCLC
Nikolaos Ntertsos1, George Christantoniou2, Krystallia Kyrka3, Persefoni Pezirkianidou4, Vasileios Bikos5, Papadaki Konstantina4 and Theodora Tsiouda6*
13rd Department of Clinical Oncology, ‘Theagenio’ Cancer Hospital, 54007 Thessaloniki, Greece
2Endocrinology Department, ‘Theagenio’ Cancer Hospital, 54007 Thessaloniki, Greece
3Aristotle University of Thessaloniki, Greece
4Department of Computer Science and Biomentical Informatics, University of Thessaly, Greece
5Candidate, Pulmonologist, ‘Theagenio’ Cancer Hospital, Greece
6Pulmonary‑Oncology Department, ‘Theageneio’ Cancer Hospital, Greece
*Address for Correspondence: Theodora Tsiouda, MD, MSc, PhD, Pulmonologist, Director of Pulmonary‑Oncology Department, ‘Theageneio’ Cancer Hospital, Greece, Email: [email protected]
As the introduction of immune checkpoint inhibitors in the treatment of various cancers is now proven to be already acquired knowledge, so does a new challenge arise for clinicians; the understanding, diagnosis, and management of the rarest adverse effects of immunotherapy. We present a case of type-1 diabetes Mellitus (T1DM) in a patient with non-small cell lung carcinoma (NSCLC) treated with pembrolizumab. Following ten cycles of treatment, our patient was diagnosed with T1DM after being admitted for diabetic ketoacidosis and stayed hospitalized in the ICU. Later, they continued treatment with insulin, having shown disease response to pembrolizumab, and resumed immunotherapy while on insulin. Immunotherapy-induced T1DM can sometimes occur with PD1/PD-L1 blockage therapies. It has a rapid onset, is characterized by insulin deficiency due to the autoimmune destruction of beta-cells, and usually presents itself with diabetic ketoacidosis. Unlike most of the other adverse effects of immunotherapy, glucocorticoids don’t seem to be of therapeutic value, and insulin substitution is required. Regular glucose monitoring can be key to early diagnosis and prevention of hospitalization.
Nearly 10 years since the approval of the first programmed death receptor 1/programmed death ligand-1 (PD-1/PD-L1) blocking agents (Table 1), they now have become a standard of care and incorporated into clinical practice in the management of many cancers (melanoma, lung cancer, head and neck cancers, bladder cancers, etc.).
| Table 1; Overview of Major FDA-Approved PD-1/PD-L1 Inhibitors. | ||||
| Drug name | Commercial name | Target | Approval year | Cancers |
| Nivolumab | Opdivo | PD-1 | 2014 | melanoma, NSCLC, mesothelioma, renal, head and neck, hepatocellular, urothelial, esophageal, gastric |
| Pembrolizumab | Keytruda | PD-1 | 2014 | melanoma, NSCLC, urothelial, renal |
| Atezolizumab | Tecentriq | PD-L1 | 2016 | SCLC, hepatocellular, NSCLC, urothelial |
| Avelumab | Bavencio | PD-L1 | 2017 | urothelial, renal, Merkel-cell |
| Durvalumab | Imfinzi | PD-L1 | 2017 | urothelial, NSCLC, biliary |
| Cemiplimab | Libtayo | PD-1 | 2018 | skin, NSCLC |
| Dostarlimab | Jemperli | PD-1 | 2021 | endometrial |
| Retifanlimab | Zynyz | PD-1 | 2023 | Merkel-cell |
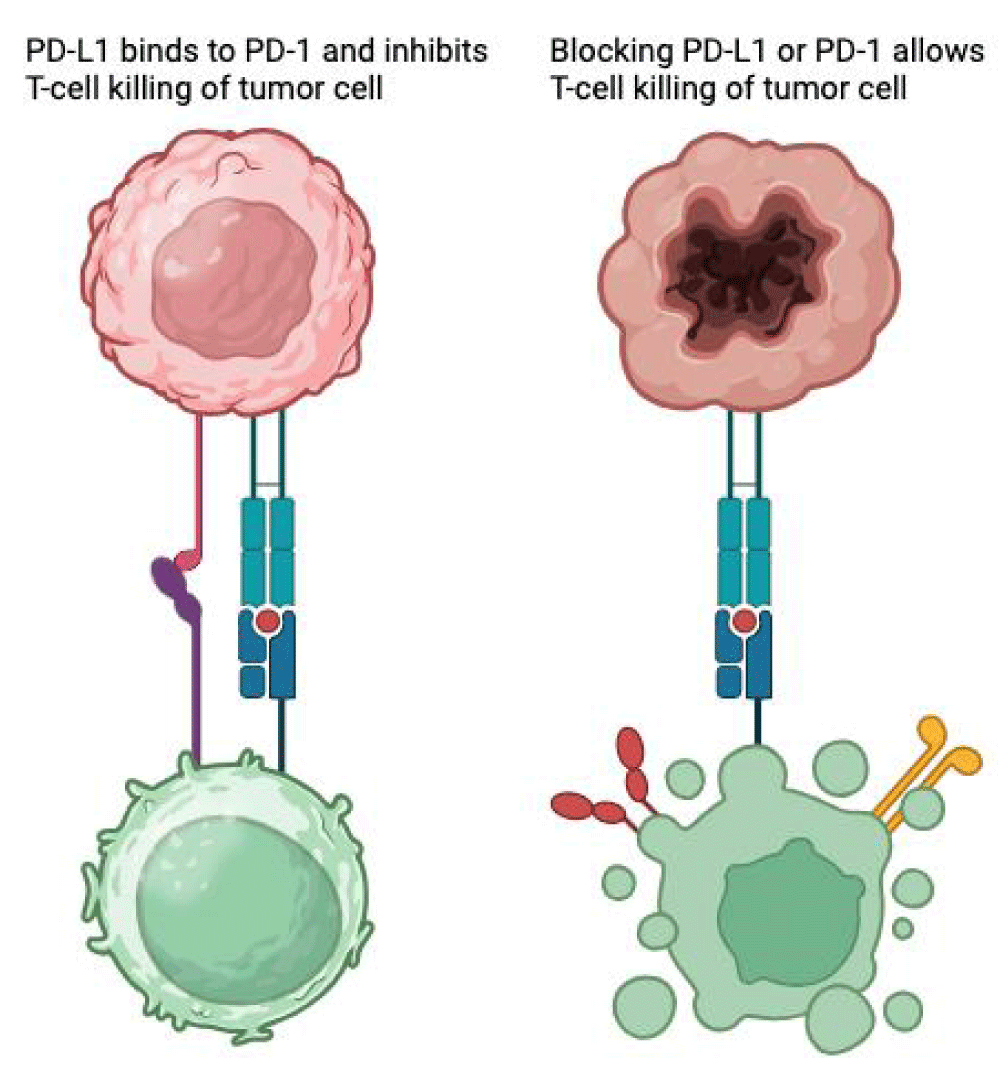
PD-1 is a transmembrane checkpoint protein located on the surface of T-cells and other leukocytes, functioning as a “break”, regulating their activation and reducing unwanted damage to healthy tissues. When binding to its ligand, PD-L1 - expressed by cancer cells - serves as a tumor’s survival measure, suppressing T-cell mediated cytotoxicity by turning them into regulatory T-cells or increasing apoptosis. PD-1/PD-L1 inhibition enhances T-cell proliferation, activation, and survival, leading to tumor cell destruction [1-3] (Figure 1).
Figure 1: PD-L1/PD-1 Blockade Enhances T-Cell-Mediated Tumor Cell Killing.
This general immunologic enhancement seems to be the cause of immune-related adverse effects (irAEs), in this case, inflammation and destruction of pancreatic beta-cells located on the islets of Langerhans.
A 45-year-old male with no comorbidities and a smoking history (60 pack years), sought medical attention because of lower respiratory tract symptoms and fever. His chest X-ray showed a tumor on the right upper lobe of the lung. Further imaging with computed tomography of the chest/abdomen, brain MRI, and PET-CT revealed findings of metastatic disease, consisting of a tumor on the right upper lung, multiple secondary nodular lesions on both lungs, a secondary lesion on the left adrenal gland and peritoneal carcinomatosis. A CT-guided biopsy of the tumor confirmed the diagnosis of a NOS, non-small-cell lung carcinoma (NSCLC), with a high PDL-1 score (TPS: 60%). Due to the high expression of PDL-1, mono-therapy with pembrolizumab was initiated.
His lab results, following induction to immunotherapy, were within normal ranges, with a fasting serum glucose level range of 68-123 mg/dl. After 6 months of systemic therapy and after being submitted to the tenth infusion of pembrolizumab, our patient had to be urgently admitted to the emergency room in a comatose state, Glasgow scale 8/15 and systolic blood pressure 65/42 mmHg. Lab tests showed acute kidney injury (urea = 121 mg/dl, creatinine = 1.97 mg/dl), metabolic acidosis (pH = 6.7, bicarbonate levels = 12 mEq/L, pCO2 = 21 mmHg), serum glucose at 693 mg/dl and HbA1c value at 6.1%. He was diagnosed with diabetic ketoacidosis and was transferred to the Invasive Care Unit (ICU). The patient’s regimen included intravenous fluid replacement, intravenous short-acting insulin, and potassium replenishment and needed non-invasive ventilation. The patient was then discharged after 30 days with an HbA1c value of 6.2%. An immunologic blood test for auto-immune diabetes turned out negative (anti-GAD, anti-insulin antibodies).
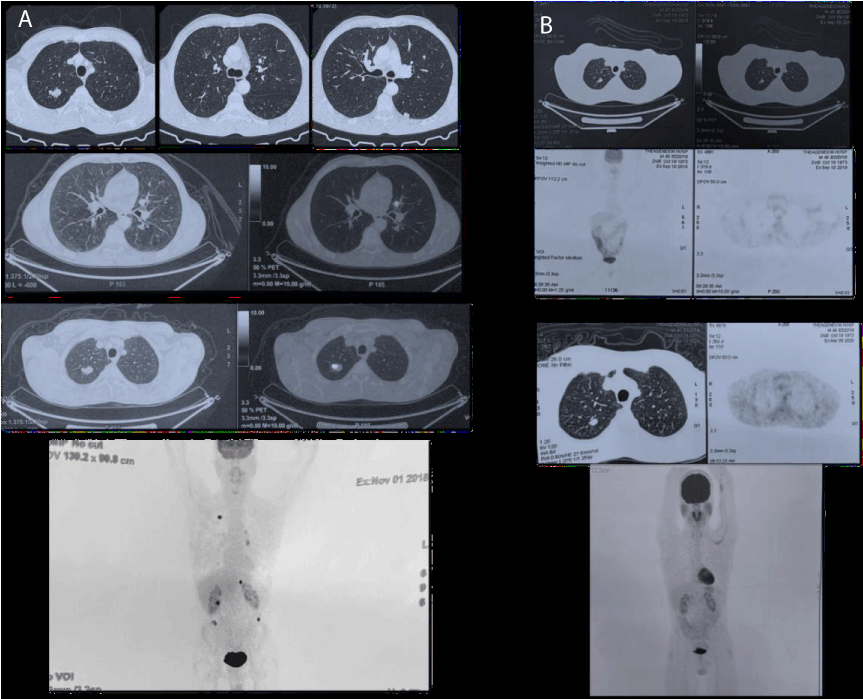
His next follow-up with PET-CT showed a complete metabolic response to pembrolizumab and the continuation of immunotherapy was decided, under endocrinologic consultation and insulin substitution with a basal-bolus insulin regimen, while his HbA1c values varied from 7.2% - 8% Figure 2.
Figure 2: A) Before immunotherapy: Suspicious finding in the right upper lobe of the lung, multiple bilateral lung nodules present, suspicious finding in the left adrenal gland; possible secondary lesion, implantations in the sub-renal spaces bilaterally. B) 12 months on immunotherapy (upper) and 18 months on immunotherapy (lower): Reduced dimensions of the right upper lobe lesion of the lung and reduced dimensions of the bilateral lung nodules, left secondary lesion, and peritoneal implantations - which no longer indicate metabolic activity. Imaging findings of inflammation in the parotid glands bilaterally - possible epithelioid as a result of immunotherapy (lower).
At first, immunotherapy-induced T1DM was considered to be a rare occurrence, based on clinical trials. In a study in 2012, only one out of the 207 patients receiving immunotherapy checkpoint inhibitors (ICI) (0.48%) was presented with T1DM [4]. In another study with 7.551 patients from 38 clinical trials, 0.2% of the patients had developed insulin deficiency [5]. But in reality, the percentage of immunotherapy-induced DM1 is higher, as it seems in a study with 538 patients receiving anti-PD1 immunotherapy for melanoma (2015-2018), where a percentage of 1.9% appeared to have ICI-induced T1DM [6]. In another study with 1444 patients receiving ICI treatment, 12 (0.8%) developed insulin-deficient diabetes. A case series, reviewed over a 6-year period at two academic institutions, showed that 27 patients who developed T1DM, accounting for 0.9% of all these patients, received either anti-PD-1 or anti-PD-L1 antibodies treatment [7].
Up until 2020, a total of 103 patients developed ICI-induced T1DM. The age range was between 28 and 87 years old. 58 patients were male and 31 female. For the remaining 14 patients, information about their gender was not available. 93% were treated with anti-PD1 and the rest with anti-PD-L1 agents [8]. The duration from ICI to hyperglycemia ranged from 5 days to 23 months (1-27 cycles of ICI). Due to the rapid pancreatic beta-cell destruction, patients develop rapidly type-1 diabetes and are primarily diagnosed because of DKA (62.1%) and are in need of hospitalization for ketoacidosis management [9]. Immunotherapy-induced DM1’s most common symptoms are the 3 P’s; polydipsia, polyphagia, and polyurea. Weakness, irritability, palpitation, heat intolerance, fruity breath smell, and blurred vision are typical symptoms, too.
Laboratory findings in T1DM could be the elevation of glucose (fasting glucose > 126 mg/dl), HbA1c (> 6.5%), and high serum amylase levels (as a result of pancreatic inflammation). C-peptide levels are low at first but, quickly, it almost reduces to zero due to the inability of the pancreas to produce insulin. 32.9% of the patients were found to have positive auto-antibodies associated with T1DM (islet-related antibodies, anti-ICA, anti-IA, anti-GAD). In the literature, there has been a correlation between immunotherapy-induced T1DM and certain HLA types [10], meaning, possibly, that patients with genetic predisposition to T1D are more likely to get diabetes from immunotherapy, in contrast with the general population.
There are different aspects between immune checkpoint inhibitor (ICI) induced T1DM and non-ICI T1DM. Firstly, the average age of diagnosis is higher in ICI-induced T1DM, probably due to the age of cancer diagnosis. Secondly, in ICI-induced T1DM, pancreatic beta-cells are destroyed rapidly, so, insulin deficiency is more instantaneous. Also, the percentage of patients with positive auto-antibodies in ICI-induced T1DM is lower. Lastly, 60% of ICI-induced T1DM tested positive for HLA DR4 haplotypes, but in typical T1DM the percentage is much higher.
Contrary to other immune-related adverse effects (irAEs) such as hypophysitis, pneumonitis, colitis, rash, pruritus, hepatitis, and neurotoxicity, ICI-induced T1DM can’t be treated with high doses of corticosteroids (prednisolone) or immunosuppressive agents [10]. The reason isn’t clear but probably because of the already irreversible destruction of beta-pancreatic cells and the hyperglycemia following the administration of corticosteroids. Insulin is the only treatment for ICI-induced DM1, through a basal-bolus therapeutic regimen [11,12].
ICI-induced T1DM is a rare but severe adverse effect of immunotherapy. Percentages are probably expected to increase as immunotherapy use spreads in various cancer treatments. Given the rapid onset of ICI-induced T1DM and the high percentage of DKA, early diagnosis and treatment are critical to decrease mortality and improve prognosis. Therefore, physicians should be aware of impaired glucose levels; plasma glucose testing and HbA1c testing are recommended before and at each administration of anti-PD-1 or anti-PD-L1 therapy. Patients should be educated about the symptoms of hyperglycemia and ketoacidosis to raise awareness. If there is clinical suspicion of ICI-induced T1DM, HLA type, C-peptide and T1DM-associated antibodies should be tested to confirm the diagnosis. Importantly, insulin therapy should be initiated in time. Insulin treatment should be continued at home with frequent glucose testing under endocrinologic monitoring.
- Alsaab HO, Sau S, Alzhrani R, Tatiparti K, Bhise K, Kashaw SK, Iyer AK. PD-1 and PD-L1 Checkpoint Signaling Inhibition for Cancer Immunotherapy: Mechanism, Combinations, and Clinical Outcome. Front Pharmacol. 2017 Aug 23;8:561. doi: 10.3389/fphar.2017.00561. PMID: 28878676; PMCID: PMC5572324.
- Liu J, Chen Z, Li Y, Zhao W, Wu J, Zhang Z. PD-1/PD-L1 Checkpoint Inhibitors in Tumor Immunotherapy. Front Pharmacol. 2021 Sep 1;12:731798. doi: 10.3389/fphar.2021.731798. PMID: 34539412; PMCID: PMC8440961.
- Olszanski AJ. Principles of immunotherapy. J Natl Compr Canc Netw. 2015 May;13(5 Suppl):670-2. doi: 10.6004/jnccn.2015.0199. PMID: 25995426.
- Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, Pitot HC, Hamid O, Bhatia S, Martins R, Eaton K, Chen S, Salay TM, Alaparthy S, Grosso JF, Korman AJ, Parker SM, Agrawal S, Goldberg SM, Pardoll DM, Gupta A, Wigginton JM. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012 Jun 28;366(26):2455-65. doi: 10.1056/NEJMoa1200694. Epub 2012 Jun 2. PMID: 22658128; PMCID: PMC3563263.
- Barroso-Sousa R, Barry WT, Garrido-Castro AC, Hodi FS, Min L, Krop IE, Tolaney SM. Incidence of Endocrine Dysfunction Following the Use of Different Immune Checkpoint Inhibitor Regimens: A Systematic Review and Meta-analysis. JAMA Oncol. 2018 Feb 1;4(2):173-182. doi: 10.1001/jamaoncol.2017.3064. PMID: 28973656; PMCID: PMC5838579.
- Tsang VHM, McGrath RT, Clifton-Bligh RJ, Scolyer RA, Jakrot V, Guminski AD, Long GV, Menzies AM. Checkpoint Inhibitor-Associated Autoimmune Diabetes Is Distinct From Type 1 Diabetes. J Clin Endocrinol Metab. 2019 Nov 1;104(11):5499-5506. doi: 10.1210/jc.2019-00423. PMID: 31265074.
- Stamatouli AM, Quandt Z, Perdigoto AL, Clark PL, Kluger H, Weiss SA, Gettinger S, Sznol M, Young A, Rushakoff R, Lee J, Bluestone JA, Anderson M, Herold KC. Collateral Damage: Insulin-Dependent Diabetes Induced With Checkpoint Inhibitors. Diabetes. 2018 Aug;67(8):1471-1480. doi: 10.2337/dbi18-0002. Epub 2018 Jun 24. PMID: 29937434; PMCID: PMC6054443.
- https://cdn-links.lww.com/permalink/cm9/a/cm9_2020_06_08_cai_cmj-2020-382_sdc2.pdf
- Imagawa A, Hanafusa T, Miyagawa J, Matsuzawa Y. A novel subtype of type 1 diabetes mellitus characterized by a rapid onset and an absence of diabetes-related antibodies. Osaka IDDM Study Group. N Engl J Med. 2000 Feb 3;342(5):301-7. doi: 10.1056/NEJM200002033420501. PMID: 10655528.
- Clotman K, Janssens K, Specenier P, Weets I, De Block CEM. Programmed Cell Death-1 Inhibitor-Induced Type 1 Diabetes Mellitus. J Clin Endocrinol Metab. 2018 Sep 1;103(9):3144-3154. doi: 10.1210/jc.2018-00728. PMID: 29955867.
- Aleksova J, Lau PK, Soldatos G, McArthur G. Glucocorticoids did not reverse type 1 diabetes mellitus secondary to pembrolizumab in a patient with metastatic melanoma. BMJ Case Rep. 2016 Nov 23;2016:bcr2016217454. doi: 10.1136/bcr-2016-217454. PMID: 27881588; PMCID: PMC5174822.
- Smati S, Buffier P, Bouillet B, Archambeaud F, Vergès B, Cariou B. Expert opinion on immunotherapy induced diabetes. Ann Endocrinol (Paris). 2018 Oct;79(5):545-549. doi: 10.1016/j.ando.2018.07.006. Epub 2018 Jul 25. PMID: 30126628.