More Information
Submitted: June 17, 2024 | Approved: July 01, 2024 | Published: July 02, 2024
How to cite this article: Wu C, Mendez G, Gandhi A, Kambhatla S, Siddiqui F, et al. An Interesting Case of COPD Exacerbation Presenting with Mixed Features of Intracranial Hypertension and Hypercapnic Encephalopathy. J Pulmonol Respir Res. 2024; 8: 035-041.
DOI: 10.29328/journal.jprr.1001056
Copyright License: © 2024 Wu C, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Hypercapnia; Pulmonary hypertension; Intracranial venous hypertension; Idiopathic intracranial hypertension; Papilledema; Intractable headache
Abbreviations: IIH: Intracranial Hypertension; IVH: Intracranial Venous Hypertension; LP: Lumbar Puncture; Ophthalmology Exam; COPD: Chronic Obstructive Pulmonary Disease; Papilledema
An Interesting Case of COPD Exacerbation Presenting with Mixed Features of Intracranial Hypertension and Hypercapnic Encephalopathy
Chaoneng Wu1*, Gustavo Mendez2, Aaron Gandhi11, Sujata Kambhatla1, Furqan Siddiqui3, Amin Pasha3 and Ramesh Madhavan2*
1Internal Medicine Department, Garden City Hospital, Michigan State University, 6245 Inkster Rd, Garden City, MI 48135, USA 2Neurology Department, Garden City Hospital, Michigan State University, 6245 Inkster Rd, Garden City, MI 48135, USA 3Pulmonology and Critical Care Department, Garden City Hospital, Michigan State University, 6245 Inkster Rd, Garden City, MI 48135, USA
*Address for Correspondence: Dr. Ramesh Madhavan, Neurology Department, Garden City Hospital, Michigan State University, 6245 Inkster Rd, Garden City, MI 48135, USA, Email: [email protected]
Dr. Chaoneng Wu, Internal Medicine Department, Garden City Hospital, Michigan State University, 6245 Inkster Rd, Garden City, MI 48135, USA, Email: [email protected]
Background: Idiopathic intracranial hypertension (IIH or pseudotumor cerebri) has two major morbidities: papilledema with visual loss and disabling headache. Intracranial Venous Hypertension (IVH) is a fundamental mechanism of IIH. Although traditionally considered limiting to the central nervous system, evidence suggests IIH as a systemic disease associated with cardiorespiratory disorders, which has been far less comprehended.
Case Report: A 60-year-old female with Chronic Obstructive Pulmonary Disease (COPD) was admitted for dyspnea and developed a coma with a pH of 7.01 and pCO2 of 158 mmHg. She was intubated and had persistent nuchal rigidity, a brief myoclonus episode with a negative electroencephalogram, and negative CT head studies. A Lumbar Puncture (LP) revealed elevated opening pressure (35 cmH2O) with normal Cerebral Spinal Fluid (CSF) studies. Her nuchal rigidity improved after the removal of 40 mL CSF. The ophthalmology examination the next day after her the large volume LP didn’t show visual loss or papilledema. The patient improved clinically and was extubated two days later. Her echocardiogram showed a dilated right ventricle with pulmonary hypertension. The patient was discharged home.
Discussion: IIH is different from hypercapnic encephalopathy and characterized by increased intracranial pressure with papilledema, vision loss, and debilitating headache. Hypercapnia-induced increased intracranial venous flow and pulmonary hypertension-caused elevated central venous pressure with consequent outflow resistance lead to IVH. In hypercapnic encephalopathy, the presentation is mostly cognitive changes. In this case, nuchal rigidity with a negative CT head scan triggered the investigation of IIH.
Conclusion: A deep understanding of the relationship between COPD and IIH is vital. There is insufficient evidence to recommend routine eye examinations in COPD patients for papilledema and to conduct a pulmonary function test for a newly diagnosed IIH patient. However, we highly suggest a timely ophthalmology exam prior to performing an LP in COPD patients with suspecting IIH to avoid unnecessary procedures and meanwhile improve clinical outcomes.
Idiopathic Intracranial Hypertension (IIH), previously known as pseudotumor cerebri, is characterized by increased intracranial pressure with papilledema leading to a risk of visual loss and chronic disabling headache [1]. The diagnostic criteria for IIH include (1) a normal neurological exam except for sixth cranial nerve abnormalities; (2) neuroimaging excluding structural lesion, meningeal enhancement, and hydrocephalus; (3) normal Cerebrospinal Fluid (CSF) constituents; and (4) an opening pressure greater than 25 centimeters of water (cmH2O) measured through Lumbar Puncture (LP) [2,3]. The incidence of IIH has a high degree of heterogeneity across different studies and countries. A study showed that the incidence and prevalence of women in 2017 in the UK were 9.3/100,000 and 79/100,000 per year, respectively, with women of productive age and obesity having the highest incidence (16.5/100,000 yearly) [4,5]. The incidence of IIH has been increasing which parallels the increased prevalence of obesity worldwide. Alongside elevated incidence, IIH-induced hospital admissions and financial burden to our society have been rising.
Traditionally, IIH was considered a disorder limited to the central nervous system and neuro-ophthalmic axis. However, recent studies suggested that certain systemic disorders are associated with Intracranial Venous Hypertension (IVH) leading to IIH [6]. IVH is a primary mechanism and the “final common pathway” for IIH. Based on the pathological drivers, IVH is stratified into 4 groups: (1) Central Venous Pressure (CVP) -mediated elevations in cerebral venous pressures; (2) cerebral venous stenosis- mediated; (3) a combination of both and (4) post-thrombosis syndrome [7]. CVP-mediated IVH is related to cardiorespiratory disease or obesity and accounts for approximately 25% of the total IIH cases [8].
Although the concept of obesity-related elevation in intraabdominal pressure and rising CVP leading to IIH has been widely accepted, however, there is much less awareness of cardiorespiratory disease-associated IVH. Retrospective cohort studies reported Obstructive Sleep Apnea (OSA) as the risk factor for neuro-ophthalmic disorders occurred in 48% to 60% of IIH patients, proposing a strong association between the two conditions [9,10]. Another case series study also reported that 9 out of 16 patients with IIH had asthma or reactive airway diseases (56%) [11]. Currently, no report can be found about acute exacerbation of chronic obstructive pulmonary disease (ECOPD) causing IIH. Here we report a case who presented with mixed features of IIH and hypercapnic encephalopathy (HE) in the setting of ECOPD. Information from this study may provide insight into the link between hypercapnia and Pulmonary Hypertension (PH)-induced CVP causing IVH and IIH, which aids in diagnosing and managing IIH in cardiorespiratory disorders, a typical but far under-recognized entity.
A 60-year-old female presented with exertional dyspnea for one week that worsened on the day of admission. She had a past medical history of emphysema and Rheumatoid Arthritis (RA). She was hospitalized twice over the past 6 months due to ECOPD and discharged with a Trelegy Ellipta (fluticasone-umeclidin-vilanter) inhaler without home oxygen. Her end-stage RA caused symmetrically deformed joints in both hands. She was noticed to have excessive daytime sleepiness for a few weeks by her son. A review of systems showed no fever, headaches, chills, coughs, sputum production, or chest pain. She had no history of pulmonary embolism, deep vein thrombosis, cerebrovascular accident, or heart attack. She reported a history of bilateral otitis media without perforation about a month earlier and received treatment with doxycycline.
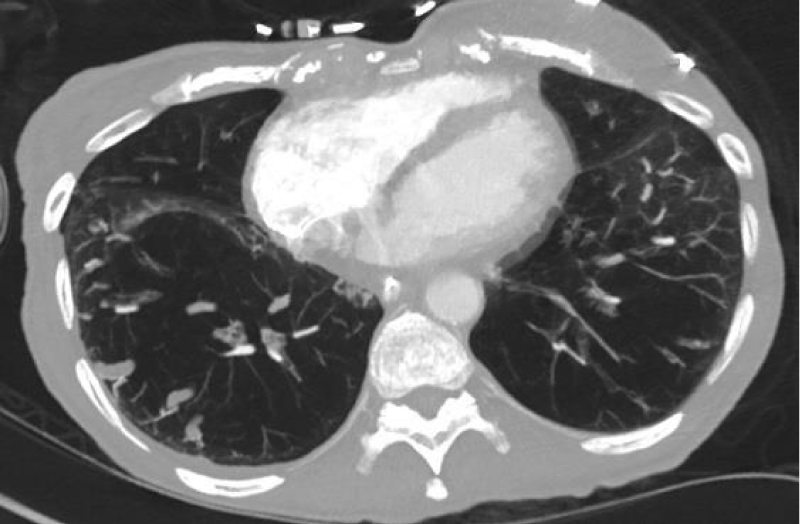
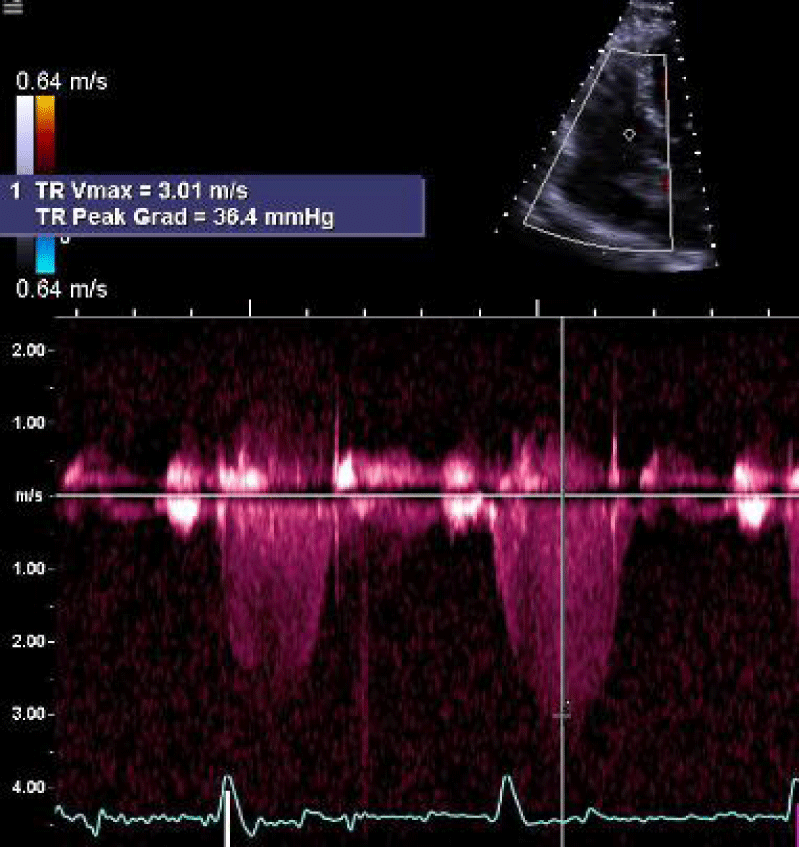
On arrival, vital signs were temperature 36.5 oC, heart rate 66, respiratory rate 18, blood pressure 109/59 mmHg, and oxygen saturation of 94% on 3 liters of oxygen. Physical exam showed thin body habitus, mildly increased breathing efforts, positive jugular vein distention (JVD), expiratory wheezing sounds in the posterior lung fields, regular heartbeats without murmurs, and bilateral pitting edema at her ankles. Laboratory work showed elevated bicarbonate (32.3 mEq/L) but otherwise unremarkable (Table 1). She was diagnosed with ECOPD and treated with methylprednisolone, budesonide, and ipratropium bromide/albuterol nebulizers. A Computed Tomography (CT) scan with contrast showed emphysema without pulmonary embolism (Figure 1). An echocardiogram showed a Right Ventricular Systolic Pressure (RVSP) of 45 mmHg with moderate tricuspid valve regurgitation (Figure 2).
Figure 1: Computed Tomography (CT) scan of the lungs. A CT scan showed diffuse emphysema in the lower lobes of both lungs.
Figure 2: An echocardiogram showed Right ventricular systolic pressure (RVSP) of 45 - 50 mmHg and moderate tricuspid regurgitation with tricuspid valve regurgitation velocity of 3.01 m/s.
| Table 1: Laboratory data. | |||
| Laboratory data | Day 1 | Day 2 | Normal range |
| White-cell count (per µL) | 9.7 | 6.3 | 3.5 -10.5 |
| Hemoglobin (g/dL) | 15.9 | 13.9 | 12 -15.5 |
| Hematocrit (%) | 49.6 | 41.8 | 35 - 45 |
| Platelet count (per µL) | 255 | 203 | 150 - 450 |
| Absolute Neutrophils (103/μL) | 9.0 | 8.8 | 1.7 - 7.0 |
| Absolute Eosinophils (103/μL) | 0.1 | 0.1 | 0 |
| Glucose (mg/dL) | 126 | 89 | 70 -140 |
| Sodium (mmol/liter) | 143 | 144 | 135 -145 |
| Potassium (mmol/liter) | 5.4 | 3.4 | 3.5 - 5.3 |
| Chloride (mmol/liter) | 108 | 99 | 98 - 110 |
| Carbon dioxide (mmol/liter) | 32.3 | 34.8 | 20 - 28 |
| Angion gap | 4.7 | 4.5 | 6 -10 |
| Urea nitrogen (mg/dL) | 23.0 | 14 | 6 - 24 |
| Creatinine (mg/dL) | 0.61 | 0.56 | 0.5 - 1.0 |
| Aspartate aminotransferase (units/L) | 19 | 18 | 10 - 36 |
| Alanine transaminase (units/L) | 25 | 20 | 6 - 29 |
| Alkaline Phosphatase (IU/L) | 75 | 56 | 33 - 130 |
| Total bilirubin (mg/dL) | 0.7 | 0.5 | 0.2 - 1.2 |
On day 2, she became drowsy with intermittent confusion and disorientation but could be easily reoriented. Her vital signs were stable and laboratory workup did not show major changes other than an increase in bicarbonate to 34.8(Table 1). She was considered to have hospital-induced delirium and no medication changes were made. On that night, a rapid response was called as she had become comatose with a Glasgow Coma Scale of 8 points and weak cough and gag reflexes. Her vital signs were stable with glucose of 120 mg/dL. An Arterial Blood Gas (ABG) revealed a pH of 7.01, pCO2 158 mmHg, O2 87 mmHg, and bicarbonate over 40 mEq/L. She was intubated for respiratory failure and airway protection and received fentanyl for sedation and pain management.
Several hours after her intubation, she had a 20-second episode of right lower leg jerking movement. A neurologic exam revealed nuchal rigidity with no other focal neurological deficit. A ceribell electroencephalogram (EEG) for 24 hours didn’t show seizure activities. A CT head scan showed no acute intracranial changes. A spinal tap was performed on day 3 in consideration of meningoencephalitis or subarachnoid hemorrhage. The opening pressure was 35 cmH2O. After removing 40 mL of CSF, the closing pressure reading was 11 cmH2O. The CSF study showed clear fluid with normal cytology, chemistry, and microbiology tests (Table 2).
| Table 2: Cerebral spinal fluid analysis. | ||
| CSF | Test | Normal range |
| Color | Transparent | Transparent |
| Opening pressure (cmH2o) | 35 | < 20 |
| White blood cell count (per mm3) | 2 | < 5 |
| Cell differential Mononuclear cells (%) |
80% | - |
| Glucose (mg/dL) | 70 | 40-80 |
| Protein (mg/dL) | 24.6 | 15-60 |
| Bacterial culture | Negative | Negative |
| West Nile IgM, IgG | Negative | Negative |
| VDRL | Negative | Negative |
| Lyme IgM, IgG | Negative | Negative |
| *The opening pressure was down to 11 cmH2o after tapping 40 mL of CSF. | ||
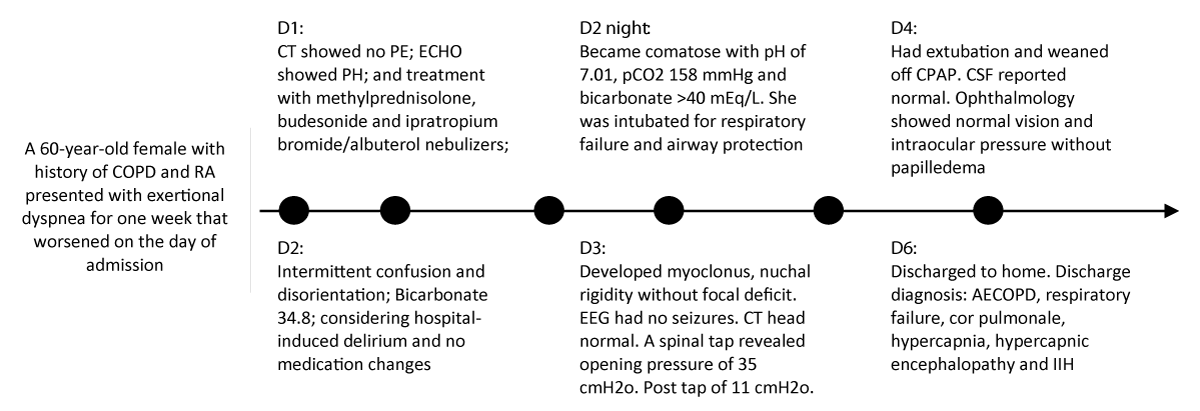
On day 4 of her admission, the ventilator settings were minimal. An ABG study showed a pH of 7.34, pCO2 59 mmHg, O2 71 mmHg, and bicarbonate 30mEq/L. She was successfully extubated and put on continuous positive airway pressure for 12 hours. Ophthalmology was consulted for papilledema evaluation on day 4. The patient denied any changes in vision or diplopia. Her exam revealed normal visual acuity; no afferent pupillary defect in both eyes; and normal intraocular pressure (IOP: 15 mm Hg OD/right eye and 18 mm Hg OS/left eye). Examination of the optic discs didn’t show marked optic nerve swelling or papilledema. The patient was discharged home on day 6 with a diagnosis of ECOPD, respiratory failure, cor pulmonale, hypercapnia, Hypercapnic Encephalopathy (HE), and IIH. The key clinical events and findings are listed in Figure 3.
Figure 3: Key Clinical Events and Findings.
This case had mixed features of IIH and HE as the complications of ECOPD. IIH is characterized by increased intracranial pressure including morbidities of papilledema with increased risk of vision loss and chronic debilitating headache. In contrast, the symptoms of HE are majorly psychomotor agitation, cognitive defects, confusion with asterixis, delirium, and coma [12]. The principal pathological mechanism for IIH is deregulated venous blood flow with resultant IVH. In HE, the predominant mechanism is different, with the presence of brain edema due to cerebral hypoxia and acidosis with deranged neurotransmitters [13,14]. This patient was comatose and had transient myoclonus due to significant hypercapnia (CO2 of 135 mmHg) secondary to ECOPD, indicating the development of HE [15]. After mechanical ventilation support and other treatments, her symptoms of HE improved within two days. However, the patient presented with persistent neck stiffness which was not a usual finding in HE. She was afebrile and had normal white blood cell counts; negative Kernig’s and Brudzinski’s signs and normal brain CT scan. Nevertheless, her recent history of bilateral otitis media and potential impaired immune function secondary to RA warranted ruling out meningitis or infectious encephalopathy. An LP was therefore performed that revealed an opening pressure of 35 cmH2O with normal CSF analysis. For this, she was diagnosed with IIH in the setting of ECOPD.
In literature, the strong link between OSA, asthma, respiratory reactive disease, and IIH has been suggested [16]. For example, a retrospective cohort study investigated 110 patients with IIH and revealed 48.6% of patients had OSA based on overnight pulse oximetry screening tool [10]. Another two case series studies revealed the occurrence of 60% and 33.3% of OSA in IIH according to the patient’s self-reported OSA history [17,18]. Currently, no studies can be found that ECOPD causes IIH. Our article is therefore of great significance to firstly reveal the possible link between ECOPD and IIH.
How does IIH occur in ECOPD? Pathologically, IVH is the fundamental and “final common pathway” for IIH. Based on Monro-Kellie doctrine, an alteration in CSF volume, blood, and brain leads to reciprocal changes in one or the other two to maintain the equilibrium of intracranial pressure (ICP) [19]. Furthermore, the blood volume dynamics weigh much higher than the other two [20]. With ECOPD, hypercapnia(PaCO2 ≥ 45 mmHg) results in cerebral vasodilation and increased cerebral blood volume (CBV) which eventually causes rising venous flow volume [21,22]. A study showed that PaCO2 > 80 mm Hg increases CBV up to 6 times its baseline [22]. Obstructive sleep apnea (OSA) is also associated with increased ICP secondary to hypercapnia and acidosis due to cerebral vasodilation and increased vascular permeability [23]. On the other hand, hyperventilation therapy lowers CO2 levels with consequently dropped CBV and ICP, and therefore benefits post-traumatic intracranial hypertension [24,25]. Our patient had a CO2 level of 158 mmHg, which constituted one primary pathogenesis in IVH due to venous hyperemia.
Additionally, the venous outflow restriction due to PH-induced CVP elevation could be another critical factor in developing IVH. This patient had JVD, lower leg pitting edema, and elevated RVSP in the ECHO study, which suggested a CVP elevation. It’s suggested that CVP and ICP pressures are coupled, which means the alterations in one usually parallel those in the others [26,27]. For example, a study revealed a strong positive linear relationship between CVP and ICP, and further, breathing through inspiratory resistance decreased both CVP and ICP [28]. Accordingly, in our case, the elevated CVP could be retrogradely transmitted to ICP once it prevails over the venous compliance (change in volume relating to the change in pressure, ΔV/ΔP) [29,30], which could also be described as a Starling resistor of the bridging veins within the skull [31]. Again, although a balanced CSF production and reabsorption is an important determinant of ICP, whether and how ECOPD stimulates the subarachnoid space remodeling involved in CSF deregulation remains unknown.
How is IIH diagnosed? Headache and visual complaints are common symptoms of IIH. Nevertheless, the key finding in our patient was nuchal rigidity. Nuchal rigidity occurs when the rising ICP is transmitted down to the spinal subarachnoid space. There are case reports of nuchal rigidity as a chief complaint in patients with IIH [32-34]. It also provokes the differentials for meningeal inflammation, such as acute bacterial meningitis, subarachnoid hemorrhage, posterior fossa tumor, and multiple sclerosis [35,36]. Brain CT can exclude space-occupying lesions, such as hydrocephalus, subarachnoid hemorrhage, mass, and cerebral venous thrombosis. Magnetic Resonance Imaging (MRI) is not necessary to diagnose IIH, but this may help with diagnosing papilledema with findings of optic disc swelling, optic nerve sheath distention, protrusion or vertical tortuosity, and posterior globe flattening [37]. MRI also helps in the exclusion of transverse sinus stenosis [38]. Spinal tapping is both diagnostic and therapeutic and is particularly helpful through a high-volume tap with the removal of CSF (usually 40 mL - 50 mL). Our patient had a normal CT head scan. 40 mL of CSF removal had an immediate effect on ICP that changed from 35 to 11 cmH2O. Her nuchal rigidity also improved on the next day.
Papilledema is an essential neuro-ophthalmologic finding and emergent morbidity of IIH. Untreated papilledema progressing to irreversible vision loss with optic atrophy occurs in 31% of patients with IIH [39], more likely in a fulminant onset situation [40]. Therefore, a timely ophthalmology exam is crucial to assess papilledema and identify immediate risk of visual loss. It includes visual acuity, pupil examination, intraocular pressure, and visual field, a dilated fundal examination to assess papilledema severity and disc swelling, and the eye fundus including optic nerve description like hemorrhages or hyperemia. Our patient received an ophthalmology exam the next day after her LP, with normal Ocular Pressure (OP) and normal fundus. The absence of papilledema was most likely due to the large volume tap with transient resolution of IIH. Studies have shown that an OP > 25 cmH2O in the setting of IIH dramatically increases the chance of coexisting intracranial venous sinus stenosis, which may be discovered under venography [41,42]. Accordingly, it’s less likely that our patient had concomitant venous sinus stenosis making further neurosurgical evaluation unnecessary.
Another significant morbidity is intractable headache, which can be debilitating if not treated appropriately [43]. The headache phenotype is highly variable mimicking other primary headache disorders, such as migraine. It manifests as a severe, throbbing headache behind both eyes and may also include pulsatile tinnitus, visual blurring, and cognitive disturbance [44]. Actually, patients with IIH may have a 6-fold increased risk of developing migraines [45].
Finally, there are other risk factors for developing IIH (Table 3). As for our patient, she needs polysomnography to evaluate sleep apnea based on her daytime sleepiness and hypercapnia. Her recent use of doxycycline might be another risk factor as tetracycline derivatives, such as doxycycline and minocycline, may cause or worsen IIH [46,47]. Studies have shown that IIH occurs usually after several weeks of continuously using tetracycline antibiotics [47]. Additionally, with an elimination half-life of less than 24 hours, the tetracycline-induced IIH often resolved in a few days to 3 weeks [48]. Our patient took doxycycline 5 weeks prior to her admission and only for 5 days, which made it unlikely to cause her IIH. There are other situations, such as weight gain, obesity, Cushing’s disease, chronically using steroids and hypervitaminosis, but none of these fit our patient’s situation.
| Table 3: Risk factors for developing idiopathic intracranial hypertension. | |
| Drugs | Systematic disorders |
| Antibiotics: Tetracycline and derivatives | Respiratory: OSA, COPD |
| Vitamin A derivatives: isotretinoin, all-trans-retinoic acid | Cardiovascular: Superior vena cava syndrome; pulmonary hypertension |
| Corticosteroids | Endocrinology: Obesity, POCS, Cushing’s disease |
| Others: Lithium, cimetidine | Hematology: Cerebral venous sinus thrombosis; |
| Note: OSA: Obstructive Sleep Apnea; COPD: Chronic Obstructive Pulmonary Disease; POCS: Polycystic Ovarian Syndrome | |
In summary, when COPD patients present with neurological symptoms, one needs to differentiate HE from IIH. Compared to HE, IIH is a far under-recognized complication of ECOPD induced by hypercapnia and elevated CVP. A deep understanding of the relationship between ECOPD and IIH is vital. There is insufficient evidence to recommend routine eye examinations in COPD patients for papilledema and to conduct a pulmonary function test for a newly diagnosed IIH patient. However, we highly suggest a timely ophthalmology exam prior to performing an LP in COPD patients with suspected IIH to avoid unnecessary procedures and meanwhile improve clinical outcomes.
Author’s contributions
CW and GM were involved in the conception and design. CW and PA collected pertinent literature and drafted the manuscript. AG, SK, and FS were involved in the literature analysis, manuscript discussion, and revision. RM and PA provided critical revision of the manuscript. All authors read and approved the final manuscript, and agreed to be accountable for all aspects of the work.
Ethics approval and consent to participate
This study involves human participants. We declare having received the patient’s consent for the case and its publication.
- Mollan SP, Davies B, Silver NC, Shaw S, Mallucci CL, Wakerley BR, Krishnan A, Chavda SV, Ramalingam S, Edwards J, Hemmings K, Williamson M, Burdon MA, Hassan-Smith G, Digre K, Liu GT, Jensen RH, Sinclair AJ. Idiopathic intracranial hypertension: consensus guidelines on management. J Neurol Neurosurg Psychiatry. 2018 Oct;89(10):1088-1100. doi: 10.1136/jnnp-2017-317440. Epub 2018 Jun 14. PMID: 29903905; PMCID: PMC6166610.
- Wakerley BR, Mollan SP, Sinclair AJ. Idiopathic intracranial hypertension: Update on diagnosis and management. Clin Med (Lond). 2020 Jul;20(4):384-388. doi: 10.7861/clinmed.2020-0232. PMID: 32675143; PMCID: PMC7385768.
- Friedman DI, Liu GT, Digre KB. Revised diagnostic criteria for the pseudotumor cerebri syndrome in adults and children. Neurology. 2013 Sep 24;81(13):1159-65. doi: 10.1212/WNL.0b013e3182a55f17. Epub 2013 Aug 21. PMID: 23966248.
- Miah L. et al. 204 Idiopathic intracranial hypertension in Wales: population characterisation, epidemiological trends and healthcare utilisation. J. Neurol. Neurosurg. Psychiatry. 2022. doi:10.1136/jnnp-2022-abn.233.
- McCluskey G, Doherty-Allan R, McCarron P, Loftus AM, McCarron LV, Mulholland D, McVerry F, McCarron MO. Meta-analysis and systematic review of population-based epidemiological studies in idiopathic intracranial hypertension. Eur J Neurol. 2018 Oct;25(10):1218-1227. doi: 10.1111/ene.13739. Epub 2018 Aug 3. PMID: 29953685.
- Smit M, Werner MJM, Lansink-Hartgring AO, Dieperink W, Zijlstra JG, van Meurs M. How central obesity influences intra-abdominal pressure: a prospective, observational study in cardiothoracic surgical patients. Ann Intensive Care. 2016 Dec;6(1):99. doi: 10.1186/s13613-016-0195-8. Epub 2016 Oct 10. PMID: 27726116; PMCID: PMC5056912.
- Fargen KM. Idiopathic intracranial hypertension is not idiopathic: Proposal for a new nomenclature and patient classification. Journal of NeuroInterventional Surgery. 2020. https://doi.org/10.1136/neurintsurg-2019-015498.
- Yiangou A, Mollan SP, Sinclair AJ. Idiopathic intracranial hypertension: a step change in understanding the disease mechanisms. Nat Rev Neurol. 2023 Dec;19(12):769-785. doi: 10.1038/s41582-023-00893-0. Epub 2023 Nov 13. PMID: 37957260.
- Thurtell MJ, Bruce BB, Rye DB, Newman NJ, Biousse V. The Berlin questionnaire screens for obstructive sleep apnea in idiopathic intracranial hypertension. J Neuroophthalmol. 2011 Dec;31(4):316-9. doi: 10.1097/WNO.0b013e31821a4d54. PMID: 21537196; PMCID: PMC3433717.
- Kok LT, Gnoni V, Muza R, Nesbitt A, Leschziner G, Wong SH. Prevalence and utility of overnight pulse oximetry as a screening tool for obstructive sleep apnoea in newly diagnosed idiopathic intracranial hypertension. Eye (Lond). 2023 Feb;37(3):537-542. doi: 10.1038/s41433-022-01971-1. Epub 2022 Feb 24. PMID: 35210570; PMCID: PMC8867690.
- Pepin SM. Salcone EM. Idiopathic intracranial hypertension and asthma: Evidence for an association. Invest. Ophthalmol. Vis. Sci. 2004; 45:1607.
- Scala R. Hypercapnic encephalopathy syndrome: a new frontier for non-invasive ventilation? Respir Med. 2011 Aug;105(8):1109-17. doi: 10.1016/j.rmed.2011.02.004. Epub 2011 Feb 26. PMID: 21354774.
- Remzső G, Németh J, Tóth-Szűki V, Varga V, Kovács V, Domoki F. NMDA attenuates the neurovascular response to hypercapnia in the neonatal cerebral cortex. Sci Rep. 2019 Dec 11;9(1):18900. doi: 10.1038/s41598-019-55468-1. PMID: 31827200; PMCID: PMC6906464.
- ROBIN ED. Abnormalities of acid-base regulation in chronic pulmonary disease, with special reference to hypercapnia and extracellular alkalosis. N Engl J Med. 1963 Apr 25;268:917-22. doi: 10.1056/NEJM196304252681703. PMID: 13974389.
- Van Yperselle de Striho, Brasseur L, De Coninck JD. The "carbon dioxide response curve" for chronic hypercapnia in man. N Engl J Med. 1966 Jul 21;275(3):117-22. doi: 10.1056/NEJM196607212750301. PMID: 5943727.
- Farahvash A, Micieli JA. Neuro-Ophthalmological Manifestations of Obstructive Sleep Apnea: Current Perspectives. Eye Brain. 2020 Jul 7;12:61-71. doi: 10.2147/EB.S247121. PMID: 32753994; PMCID: PMC7353992.
- Fraser JA, Bruce BB, Rucker J, Fraser LA, Atkins EJ, Newman NJ, Biousse V. Risk factors for idiopathic intracranial hypertension in men: a case-control study. J Neurol Sci. 2010 Mar 15;290(1-2):86-9. doi: 10.1016/j.jns.2009.11.001. Epub 2009 Nov 30. PMID: 19945715; PMCID: PMC2815168.
- Thurtell MJ, Trotti LM, Bixler EO, Rye DB, Bliwise DL, Newman NJ, Biousse V, Bruce BB. Obstructive sleep apnea in idiopathic intracranial hypertension: comparison with matched population data. J Neurol. 2013 Jul;260(7):1748-51. doi: 10.1007/s00415-013-6858-6. Epub 2013 Feb 15. PMID: 23412355; PMCID: PMC3707935.
- Weed LH. Some Limitations Of The Monro-Kellie Hypothesis. Arch. Surg. 1929. doi:10.1001/archsurg.1929.01140130137006.
- Chesnut RM, Temkin N, Carney N, Dikmen S, Rondina C, Videtta W, Petroni G, Lujan S, Pridgeon J, Barber J, Machamer J, Chaddock K, Celix JM, Cherner M, Hendrix T; Global Neurotrauma Research Group. A trial of intracranial-pressure monitoring in traumatic brain injury. N Engl J Med. 2012 Dec 27;367(26):2471-81. doi: 10.1056/NEJMoa1207363. Epub 2012 Dec 12. Erratum in: N Engl J Med. 2013 Dec 19;369(25):2465. PMID: 23234472; PMCID: PMC3565432.
- Weinberger SE, Schwartzstein RM, Weiss JW. Hypercapnia. N Engl J Med. 1989 Nov 2;321(18):1223-31. doi: 10.1056/NEJM198911023211804. PMID: 2677729.
- Mathews AM, Wysham NG, Xie J, Qin X, Giovacchini CX, Ekström M, MacIntyre NR. Hypercapnia in Advanced Chronic Obstructive Pulmonary Disease: A Secondary Analysis of the National Emphysema Treatment Trial. Chronic Obstr Pulm Dis. 2020 Oct;7(4):336-345. doi: 10.15326/jcopdf.7.4.2020.0176. PMID: 32877962; PMCID: PMC7883913.
- Jennum P, Børgesen SE. Intracranial pressure and obstructive sleep apnea. Chest. 1989 Feb;95(2):279-83. doi: 10.1378/chest.95.2.279. PMID: 2914475.
- Zhang Z, Guo Q, Wang E. Hyperventilation in neurological patients: from physiology to outcome evidence. Curr Opin Anaesthesiol. 2019 Oct;32(5):568-573. doi: 10.1097/ACO.0000000000000764. PMID: 31211719; PMCID: PMC6735527.
- Godoy DA, Seifi A, Garza D, Lubillo-Montenegro S, Murillo-Cabezas F. Hyperventilation Therapy for Control of Posttraumatic Intracranial Hypertension. Front Neurol. 2017 Jul 17;8:250. doi: 10.3389/fneur.2017.00250. PMID: 28769857; PMCID: PMC5511895.
- Hearon CM Jr, Dias KA, Babu G, Marshall JET, Leidner J, Peters K, Silva E, MacNamara JP, Campain J, Levine BD. Effect of Nightly Lower Body Negative Pressure on Choroid Engorgement in a Model of Spaceflight-Associated Neuro-ocular Syndrome: A Randomized Crossover Trial. JAMA Ophthalmol. 2022 Jan 1;140(1):59-65. doi: 10.1001/jamaophthalmol.2021.5200. PMID: 34882176; PMCID: PMC8662537.
- Harris KM, Petersen LG, Weber T. Reviving lower body negative pressure as a countermeasure to prevent pathological vascular and ocular changes in microgravity. NPJ Microgravity. 2020 Dec 17;6(1):38. doi: 10.1038/s41526-020-00127-3. PMID: 33335101; PMCID: PMC7746725.
- Hansen AB, Lawley JS, Rickards CA, Howden EJ, Sarma S, Cornwell WK 3rd, Amin SB, Mugele H, Marume K, Possnig C, Whitworth LA, Williams MA, Levine BD. Reducing intracranial pressure by reducing central venous pressure: assessment of potential countermeasures to spaceflight-associated neuro-ocular syndrome. J Appl Physiol (1985). 2021 Feb 1;130(2):283-289. doi: 10.1152/japplphysiol.00786.2020. Epub 2020 Dec 3. PMID: 33270516.
- Wilson MH. Monro-Kellie 2.0: The dynamic vascular and venous pathophysiological components of intracranial pressure. J Cereb Blood Flow Metab. 2016 Aug;36(8):1338-50. doi: 10.1177/0271678X16648711. Epub 2016 May 12. PMID: 27174995; PMCID: PMC4971608.
- Raisis JE, Kindt GW, McGillicuddy JE, Giannotta SL. The effects of primary elevation of cerebral venous pressure on cerebral hemodynamics and intracranial pressure. J Surg Res. 1979 Feb;26(2):101-7. doi: 10.1016/0022-4804(79)90085-4. PMID: 106185.
- Luce JM, Huseby JS, Kirk W, Butler J. A Starling resistor regulates cerebral venous outflow in dogs. J Appl Physiol Respir Environ Exerc Physiol. 1982 Dec;53(6):1496-1503. doi: 10.1152/jappl.1982.53.6.1496. PMID: 6759493.
- Hassanzadeh Rad A, Aminzadeh V. Pseudotumor Cerebri Presenting by Neck Rigidity and Torticollis. Iran J Child Neurol. 2023 Winter;17(1):119-123. doi: 10.22037/ijcn.v17i1.32415. Epub 2023 Jan 1. PMID: 36721832; PMCID: PMC9881831.
- Straussberg R, Harel L, Amir J. Pseudotumor cerebri manifesting as stiff neck and torticollis. Pediatr Neurol. 2002 Mar;26(3):225-7. doi: 10.1016/s0887-8994(01)00364-2. PMID: 11955932.
- Tagoe NN, Beyuo VM, Amissah-Arthur KN. Case series of six patients diagnosed and managed for idiopathic intracranial hypertension at a tertiary institution eye centre. Ghana Med J. 2019 Mar;53(1):79-87. doi: 10.4314/gmj.v53i1.12. PMID: 31138948; PMCID: PMC6527823.
- Magliozzi R, Howell OW, Calabrese M, Reynolds R. Meningeal inflammation as a driver of cortical grey matter pathology and clinical progression in multiple sclerosis. Nat Rev Neurol. 2023 Aug;19(8):461-476. doi: 10.1038/s41582-023-00838-7. Epub 2023 Jul 3. PMID: 37400550.
- van Olst L, Rodriguez-Mogeda C, Picon C, Kiljan S, James RE, Kamermans A, van der Pol SMA, Knoop L, Michailidou I, Drost E, Franssen M, Schenk GJ, Geurts JJG, Amor S, Mazarakis ND, van Horssen J, de Vries HE, Reynolds R, Witte ME. Meningeal inflammation in multiple sclerosis induces phenotypic changes in cortical microglia that differentially associate with neurodegeneration. Acta Neuropathol. 2021 Jun;141(6):881-899. doi: 10.1007/s00401-021-02293-4. Epub 2021 Mar 29. PMID: 33779783; PMCID: PMC8113309.
- Barkatullah AF, Leishangthem L, Moss HE. MRI findings as markers of idiopathic intracranial hypertension. Curr Opin Neurol. 2021 Feb 1;34(1):75-83. doi: 10.1097/WCO.0000000000000885. PMID: 33230036; PMCID: PMC7856277.
- Chen BS, Meyer BI, Saindane AM, Bruce BB, Newman NJ, Biousse V. Prevalence of Incidentally Detected Signs of Intracranial Hypertension on Magnetic Resonance Imaging and Their Association With Papilledema. JAMA Neurol. 2021 Jun 1;78(6):718-725. doi: 10.1001/jamaneurol.2021.0710. PMID: 33871552; PMCID: PMC8056310.
- Corbett JJ, Savino PJ, Thompson HS, Kansu T, Schatz NJ, Orr LS, Hopson D. Visual loss in pseudotumor cerebri. Follow-up of 57 patients from five to 41 years and a profile of 14 patients with permanent severe visual loss. Arch Neurol. 1982 Aug;39(8):461-74. doi: 10.1001/archneur.1982.00510200003001. PMID: 7103794.
- Bouffard MA. Fulminant Idiopathic Intracranial Hypertension. Curr Neurol Neurosci Rep. 2020 Mar 26;20(4):8. doi: 10.1007/s11910-020-1026-8. PMID: 32219578.
- Zhao K, Gu W, Liu C, Kong D, Zheng C, Chen W, Li X, Liang Y, Zhou H. Advances in the Understanding of the Complex Role of Venous Sinus Stenosis in Idiopathic Intracranial Hypertension. J Magn Reson Imaging. 2022 Sep;56(3):645-654. doi: 10.1002/jmri.28177. Epub 2022 Mar 31. PMID: 35357056; PMCID: PMC9541264.
- Liu KC, Starke RM, Durst CR, Wang TR, Ding D, Crowley RW, Newman SA. Venous sinus stenting for reduction of intracranial pressure in IIH: a prospective pilot study. J Neurosurg. 2017 Nov;127(5):1126-1133. doi: 10.3171/2016.8.JNS16879. Epub 2016 Dec 23. PMID: 28009240.
- Ahmad SR, Moss HE. Update on the Diagnosis and Treatment of Idiopathic Intracranial Hypertension. Semin Neurol. 2019 Dec;39(6):682-691. doi: 10.1055/s-0039-1698744. Epub 2019 Dec 17. PMID: 31847039; PMCID: PMC7713505.
- Mitchell JL, Mollan SP, Vijay V, Sinclair AJ. Novel advances in monitoring and therapeutic approaches in idiopathic intracranial hypertension. Curr Opin Neurol. 2019 Jun;32(3):422-431. doi: 10.1097/WCO.0000000000000690. PMID: 30865008; PMCID: PMC6522204.
- Sengupta S, Eckstein C, Collins T. The Dilemma of Diagnosing Idiopathic Intracranial Hypertension Without Papilledema in Patients With Chronic Migraine. JAMA Neurol. 2019 Sep 1;76(9):1001-1002. doi: 10.1001/jamaneurol.2019.1696. PMID: 31259997.
- Koch-Weser J, Gilmore EB. Benign intracranial hypertension in an adult after tetracycline therapy. JAMA. 1967 Apr 24;200(4):345-7. PMID: 6071479.
- Winn BJ, Liao YJ, Horton JC. Intracranial pressure returns to normal about a month after stopping tetracycline antibiotics [1]. Archives of Ophthalmology. 2007. https://doi.org/10.1001/archopht.125.8.1137.
- Passi SF, Butcher R, Orme DR, Warner JEA, Stoddard GJ, Crum AV, Gouripeddi R, Kirk BH, Digre KB, Katz BJ. Increased Incidence of Pseudotumor Cerebri Syndrome Among Users of Tetracycline Antibiotics. J Neuroophthalmol. 2022 Sep 1;42(3):323-327. doi: 10.1097/WNO.0000000000001536. Epub 2022 Mar 25. PMID: 35427251; PMCID: PMC9588410.